Introduction
For many years, there has been debate about how to optimally diagnose type 1 VWD. Initial guidelines proposed that only individuals with plasma VWF levels < 30 IU/dL should be diagnosed with ‘type 1 VWD’. This was revised in recent ASH/ISTH/WFH/NHF panel which recommended that patients with a significant bleeding history and plasma VWF of 30-50 IU/dL should also be diagnosed with type 1 VWD, as opposed to the discrete ‘Low VWF’ entity proposed in previous guidelines. In this study, we investigated whether Low VWF is a discrete clinical entity, or whether it is instead part of an age-dependent type 1 VWD evolving phenotype.
Methods
We utilized datasets from two renowned national cohort studies - the Low VWF in Ireland Cohort (LoVIC) and Willebrand in the Netherlands (WiN) studies. In the LoVIC study, patients had a personal bleeding history and historically lowest VWF levels in the 30-50 IU/dL range. In the WiN study, patients had either a personal bleeding history or positive family history, combined with historically lowest VWF levels ≤ 30 IU/dL.
Based upon levels at study inclusion versus original diagnosis, WiN patients were categorized into three groups - (i) patients with persistent VWF levels <30 IU/dL; (ii) patients with partial correction in VWF levels into the range of 30-50 IU/dL; and (iii) patients with normalization of VWF levels > 50 IU/dL.
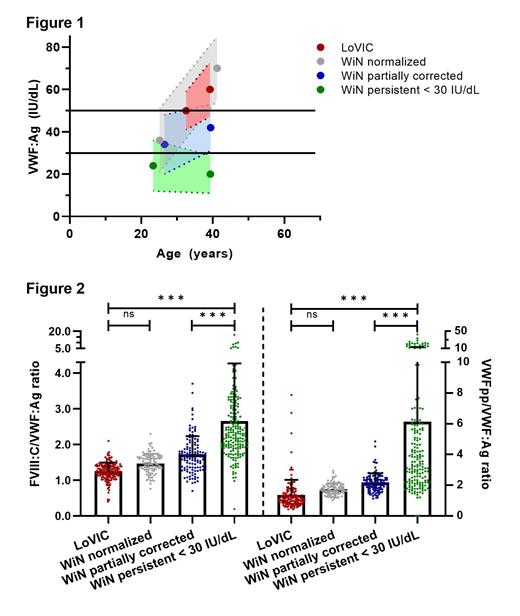
The FVIII:C/VWF:Ag ratio was used to assess the synthesis/secretion of VWF and the VWFpp/VWF:Ag ratio was used to assess the clearance of VWF.
Results
In total, 565 patients were included (403 WiN patients with type 1 VWD and 162 LoVIC patients with Low VWF). Mean age at diagnosis was significantly increased in the LoVIC cohort compared to the three WiN subgroups (32.5 years versus 23.3 years, 26.5 years and 25 years respectively; p<0.001). Conversely, there was no difference in age at time of enrollment into both national studies (p=0.532). To assess the significance of age, we first investigated how plasma VWF:Ag varied over time in WiN type 1 VWD patients compared to the LoVIC cohort. Among the total WiN cohort (with initial VWF levels < 30 IU/dL), 47% of subjects (n=188) had VWF levels that remained < 30 IU/dL despite advancing age. Conversely, 30% of type 1 VWD patients (n=121) increased their plasma VWF levels into the Low VWF range (30-50 IU/dL) over time, whereas 23% (n=94) had age-dependent increments that led to complete normalization in VWF levels > 50 IU/dL. Similarly, 39% (n=63) of patients from the LoVIC study had normalization of VWF levels over time.
Crucially, we observed that VWF:Ag in Low VWF patients clearly overlapped with those in normalized (> 50 IU/dL) type 1 VWD subjects, indicating that the LoVIC cohort is a subgroup within the WiN normalized subgroup (Figure 1). To further investigate this concept, we performed multiple regression analysis. Importantly, we observed that plasma VWF:Ag in the LoVIC cohort and the WiN normalized (> 50 IU/dL) subgroup would have not been different had they been diagnosed at the same age (difference of β=0.00 (95% CI -0.03 to 0.04)). Cumulatively, these results indicate that significant heterogeneity exists amongst type 1 VWD patients with respect to the effect of ageing on their plasma VWF levels. In addition, our findings demonstrate that because of age-dependent increases in plasma VWF:Ag, the majority of Low VWF patients would have been diagnosed with type 1 VWD had they undergone assessment earlier in life.
Consistently, no difference in prevalence of VWF mutation (36.4% vs 22.6% respectively, p=1.000), FVIII:C/VWF:Ag ratio (1.24 ±0.24 vs 1.46 ±0.27 respectively, p=0.444) or VWFpp/VWF:Ag ratio (1.36 ±0.99 vs 1.75 ±0.38 respectively, p=1.000) was found between LoVIC and normalized WiN patients (Figure 2). Contrarily, WiN patients with persistent VWF levels <30 IU/dL had more often VWF gene variants (93.0%), higher FVIII:C/VWF:Ag ratio (2.65 ±1.62) and higher VWFpp/VWF:Ag ratio (6.14 ±7.05) compared to the other groups (Figure 2, p<0.001).
Conclusion
Our findings clearly demonstrate that Low VWF does not constitute a discrete clinical or pathological entity. Rather, it is part of an age-dependent type 1 VWD evolving phenotype, as demonstrated by the same VWF levels throughout life, genetic background and pathophysiology. This study has direct consequences for VWD diagnosis, the overall understanding of type 1 VWD as an evolving disease and for patients with low VWF/type 1 VWD.
Disclosures
Atiq:Sobi: Other: Travelgrant; CSL Behring, Takeda, Octapharma and Sobi: Research Funding. Doherty:Amgen: Other: Sponsorship (Educational Support); NovoNordisk: Other: Sponsorship (Educational Support); Takeda: Honoraria. Lavin:Takeda: Honoraria, Other: Indirect funding for development of educational content, Speakers Bureau; Sobi: Consultancy, Honoraria, Speakers Bureau; Pfizer: Honoraria; Band Therapeutics: Consultancy; CSL Behring: Consultancy, Honoraria. Van Der Bom:Novo Nordisk: Research Funding. O'Connell:Freeline: Membership on an entity's Board of Directors or advisory committees; UniQure: Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sobi: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novo Nordisk: Speakers Bureau. Schols:Bayer: Research Funding. Van Galen:Amgen: Speakers Bureau; Takeda: Speakers Bureau; Bayer: Research Funding; CSL Behring: Research Funding. Fijnvandraat:Novo Nordisk: Consultancy, Research Funding; F. Hoffmann-La Roche Ltd: Consultancy; Sanofi: Consultancy; CSL Behring: Research Funding; Sobi: Consultancy, Research Funding. Baker:Bayer, AstraZeneca: Speakers Bureau; Takeda, Bristol Myers Squibb, Boehringer Ingelheim,: Research Funding; F. Hoffmann-La Roche Ltd, CSL Behring: Consultancy. Meijer:UniQure: Consultancy; Octapharma: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Alexion and CSL Behring: Speakers Bureau. James:Bayer: Research Funding; Star/Vega Therapeutics: Consultancy; Band/Guardian Therapeutics: Consultancy. Di Paola:CSL Behring: Consultancy. Eikenboom:CSL Behring: Research Funding. Leebeek:Biomarin: Consultancy; Sobi: Research Funding; CSL Behring, Takeda, UniQure: Consultancy, Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees. O'Donnell:CSL Behring: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Octapharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Leo Pharma: Speakers Bureau; Boehringer Ingelheim: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novo Nordisk: Research Funding, Speakers Bureau; Bayer: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Baxter: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Daiichi Sankyo: Membership on an entity's Board of Directors or advisory committees; Shire: Research Funding.